| Image |
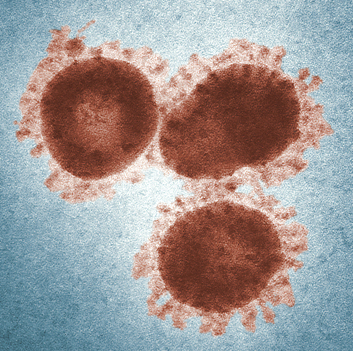 |
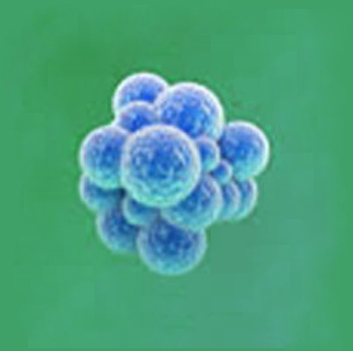 |
| Size |
0.02~0.2μm (50~100 times smaller than bacteria) |
1~5㎛ |
| Structure |
An intermediate form between living organisms and microorganisms consisting of DNA, RNA, proteins, and nucleic acids. |
Microorganisms composed of cell membrane, nucleus, DNA, and RNA materials |
| Propogation |
Parasites that need a host to live |
Independent living organisms |
| Therapy |
Antiviral / parasitic organism, so it is difficult to develop vaccines or treatments due to mutation. |
Since it is an antibiotic / complete body, it does not mutate easily, so it is easier to develop therapeutics than viruses |
| Disease incidence |
Even a small amount (10 to 100) can cause the disease. |
Disease can occur only when more than a certain amount (hundreds to millions) of bacteria are present. |
| Disease |
Influenza (flu), HIV (AIDS), Ebola (hemorrhagic fever), Corona, SARS, MERS (pneumonia), Noro (food poisoning) |
Mycobacterium tuberculosis (tuberculosis), Mycobacterium leprosy (Hansen's disease), Mycobacterium Yersinia pestis (black death), Salmonella-typhoid (food poisoning) |